quali-v®-i
FEATURES OF QUALI-V®-I CAPSULES
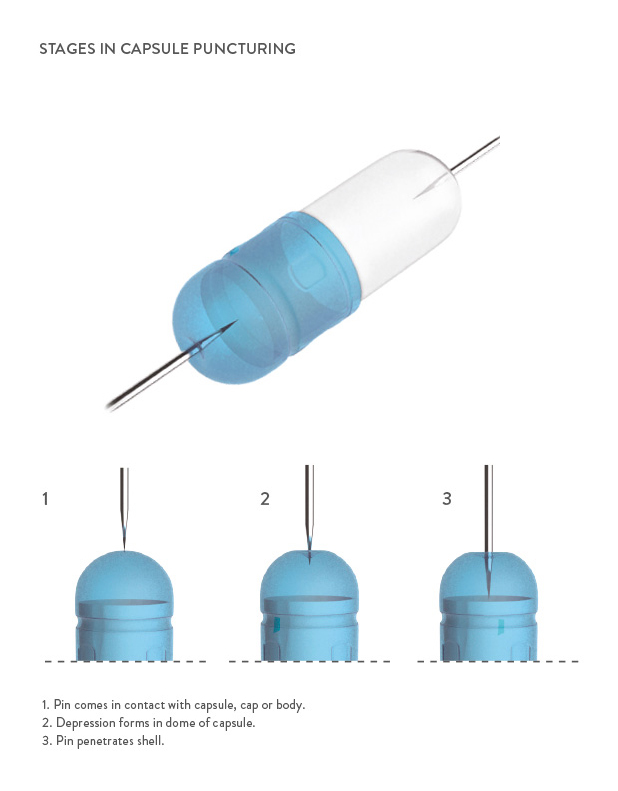
Quali-V®-I capsules have a unique composition for use in inhalation applications through DPIs. Based on Quali-V® HPMC capsules (100% plant-based), Quali-V®-I capsules are specially formulated to achieve exceptional puncturing performance, unrivalled aerosolization properties, reduced static charge and powder adhesion for more uniform dosing, and minimal microbial levels.
Qualicaps® complies with the microbiological requirements of Quali-V®-I capsules by the use of controlled manufacturing procedures that enable offering two specifications: the standard <100 cfr/g and the more stringent <10 cfr/g upon request. Strict process controls allow for these results without the use of preservatives.
Quali-V®-I does not undergo any changes in physical and chemical performance throughout its shelf life; all parameters meet the required specifications during stability studies. The GMP-compliant manufacturing process of our HPMC capsules is carried out following strict pharmaceutical criteria and certified according to ISO 9001 and 14001. Drug Master Files for the US and Canada have been registered. Quali-V®-I capsules are continuously monitored by Quality Control experts in the production process, and are also inspected using an automatic camera system to detect and remove defective units.
Available in a wide range of translucent colors (enabling the patient to verify that the capsule has been emptied and as such the dose correctly released upon inhaling) and in size 3 (the most common size in inhalation) as well as sizes 2 and 0 (for use with active ingredients that require higher doses).
Quali-V®-I capsules have the required dimensions and strength that enable them to be filled and packaged on automatic high-speed machines.
QUALI-V®-I CAPSULES, ENABLE SUPERIOR AEROsOLIZATION PARAMETERS AND MORE UNIFORM DOSING
 An important factor in powder aerosolization is the internal lubricant applied to the capsule, as it influences the quantity and consistency of powder delivery from the DPI. Since the use of an internal lubricant is essential for the capsule manufacturing process, Qualicaps® has created a specific lubricant for inhalation and carefully controls the amount administered to the stainless steel pins on which the capsule shells are formed in order to reach an optimum level.
An important factor in powder aerosolization is the internal lubricant applied to the capsule, as it influences the quantity and consistency of powder delivery from the DPI. Since the use of an internal lubricant is essential for the capsule manufacturing process, Qualicaps® has created a specific lubricant for inhalation and carefully controls the amount administered to the stainless steel pins on which the capsule shells are formed in order to reach an optimum level.
For further information, download the Quali-V®-I Scientific Review (Scientific Reviews) and the Quali-V®-I Technical Manual (Technical Manuals)